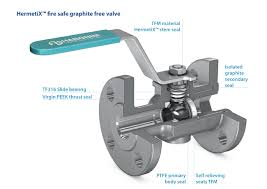
The inherent conflict for the BioPharm industry begins when defining the piping equipment to fulfill engineering
specifications. On the one side, there is a mandatory demand for fire safe certified valves in an Ex-proof zone, which
dictates graphite material as the traditional solution for stem and body seals. Then there is the counter mandatory
demand for FDA approved materials in contact with the media, accompanied by strict cleanliness demands for parts
used in high purity processes.
| Marchi | HABONIM |
|---|